Health | 07.01.2012
Europe offers contradictory advice over faulty breast implants
Germany and the Czech Republic on Friday followed France in recommending that potentially defective French-made breast implants be removed.
The German Federal Institute for Drugs and Medical Devices advised women with implants made by Poly Implant Prothèse (PIP) to have them taken out "as a precautionary measure" after evidence emerged that they may pose a potential health risk. The Czech health ministry offered similar advice to the 2,000 women in the Czech Republic believed to be at risk.
Yet Britain's Department of Health insisted on Friday that an independent review had found that there was little evidence to suggest the implants could be linked to cancer cases, although it was unclear whether they were more prone to rupture.
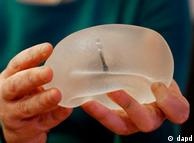
Concern over the implants, which contain industrial-grade silicone, first arose when the French government advised 30,000 women to have them removed over fears they were more prone to rupturing than standard medical implants.
 PIP sold thousands of implants in over 60 countries
PIP sold thousands of implants in over 60 countries
Germany's advice goes beyond a December 23 recommendation that women affected should have their implants checked for possible ruptures, claiming that experts had warned that silicone from such implants can leak over time even if there is no rupture.
"The urgency for removal in each case depends mainly on how long the patients have had the implants," Walter Schwerdtfeger, president of the Federal Office for Pharmaceuticals and Medical Devices, said in a statement.
Thousands affected
The institute said it was uncertain how many women received the implants in Germany. PIP claimed, however that around 300,000 implants were sold in more than 60 countries before the company went out of business in 2010. In an official investigation it was accused of seeking to save money by using an unapproved industrial silicone in some of its products rather than a medical silicone.
France has offered to pay for some 30,000 French women to have their implants removed, after more than 1,000 rupture cases.
Author: Charlotte Chelsom-Pill (AP, Reuters)
Editor: Sean Sinico
沒有留言:
張貼留言